Vaccines and Immunisation
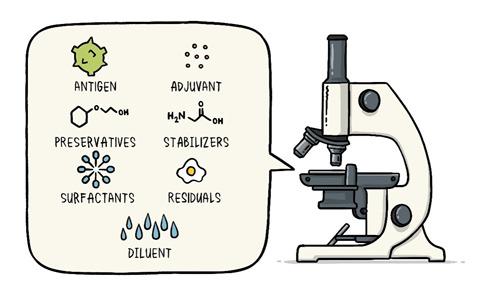
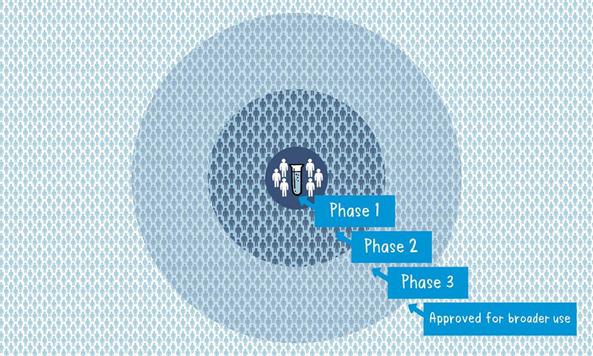
In the last article had promised for a write up on Vaccines which is the most important topic for the world now. Never the entire population of the world has waited for a vaccine like now. Interestingly during the COVID pandemic everyone has some fair idea about vaccines. While we all are eagerly for that magic COVID vaccine let’s try to understand what are these vaccines and how immunization has made the world better. Last write up I tried to explain how our body defense mechanism, Immunity works. During the COVID -19 pandemic one has seen the activities of disease prevention and control-it requires community participation, Political support and Intersectoral coordination (Health, Police, administration and others). Broadly the measures for disease prevention and control are controlling the source of infection, interrupting the routes of transmission, and protecting the susceptible host. Trace, Test and Treat were the measures adopted for controlling the source of infection. Lockdown, Quarantine, Isolation, use of mask, social distancing, hand wash and disinfection all are measures to interrupt transmission. Specific protection of susceptible hosts is possible by active Immunization. We are aware of early childhood immunization e.g. Polio, tetanus, diphtheria, measles and tuberculosis. For older people vaccine for pneumonia, flu etc. and traveler’s vaccine against yellow fever. Even vaccines are given to prevent certain cancers like the Human Papilloma Virus vaccine for the prevention of cervical cancer. The point here is that active immunization (vaccine-induced immunity) is one of the most powerful and cost-effective weapons of modern medicine. There are various immunizing agents and they may be classified as vaccines, immunoglobulins and antisera. Here we will limit to vaccines. A vaccine is an immune-biological substance designed to produce specific protection against a given disease. Vaccines are made up of viruses or bacteria that are altered or weakened so that they only cause an imitation of the disease and not the disease itself. There are a variety of different ways to alter or weaken the viruses or bacteria in vaccines so they cause the production of protective antibodies and other immune mechanisms instead of serious disease. Most viral and bacterial diseases affecting children worldwide are now preventable by Vaccine. The infant mortality rate and under 5 mortality rates (no. of children dying by their first birth and before attaining 5 years of age) respectively has come down drastically because of the mass immunization program or commonly known as Universal Immunization program. Vaccines are prepared from live modified organisms, inactivated or killed organisms, extracted cellular fractions, toxoids or a combination of these. Live modified organisms are the closest to natural infections, they are very effective but everyone cannot get them like people having weakened immune systems. Germs can travel quickly through a community and make a lot of people sick. If enough people in your community get a certain disease, it can lead to an outbreak. However, when enough people are vaccinated against a certain disease, the germs can’t travel as easily from person to person and the entire community is less likely to get the disease. Even if a person does get sick, there’s less chance of an outbreak because it’s harder for the disease to spread if a lot of people are vaccinated and therefore immune. Eventually, the disease becomes rare, and sometimes, it can be wiped out altogether, which is what happened with a very serious disease called smallpox and polio. This is known as community immunity or herd immunity. Vaccines contain tiny fragments of the disease-causing organism or the blueprints for making the tiny fragments. They also contain other ingredients to keep the vaccine safe and effective. These latter ingredients are included in most vaccines and have been used for decades in billions of doses of vaccines. Each vaccine component serves a specific purpose, and each ingredient is tested in the manufacturing process. All ingredients are tested for safety. Antigen All vaccines contain an active component (the antigen) which generates an immune response, or the blueprint for making the active component. The antigen may be a small part of the disease-causing organism, like a protein or sugar, or it may be the whole organism in a weakened or inactive form. Preservatives Preservatives prevent the vaccine from becoming contaminated once the vial has been opened if it will be used for vaccinating more than one person. Some vaccines don’t have preservatives because they are stored in one-dose vials and are discarded after the single dose is administered. The most commonly used preservative is 2-phenoxyethanol. It has been used for many years in a number of vaccines, is used in a range of baby care products and is safe for use in vaccines, as it has little toxicity in humans. Stabilizers Stabilizers prevent chemical reactions from occurring within the vaccine and keep the vaccine components from sticking to the vaccine vial. Stabilizers can be sugars (lactose, sucrose), amino acids (glycine), gelatin, and proteins (recombinant human albumin, derived from yeast). Surfactants Surfactants keep all the ingredients in the vaccine blended together. They prevent settling and clumping of elements that are in the liquid form of the vaccine. They are also often used in foods like ice cream. Residuals Residuals are tiny amounts of various substances used during the manufacturing or production of vaccines that are not active ingredients in the completed vaccine. Substances will vary depending on the manufacturing process used and may include egg proteins, yeast or antibiotics. Residual traces of these substances which may be present in a vaccine are in such small quantities that they need to be measured as parts per million or parts per billion. Diluent A diluent is a liquid used to dilute a vaccine to the correct concentration immediately prior to use. The most commonly used diluent is sterile water. Adjuvant Some vaccines also contain adjuvants. An adjuvant improves the immune response to the vaccine, sometimes by keeping the vaccine at the injection site for a little longer or by stimulating local immune cells. The adjuvant may be a tiny amount of aluminium salts (like aluminium phosphate, aluminium hydroxide or potassium aluminium sulphate). Aluminium has been shown not to cause any long-term health problems, and humans ingest aluminium regularly through eating and drinking. Each vaccine-preventable disease requires a certain percentage of people in a community to be vaccinated in order to prevent the disease’s spread. The exact percentage depends largely upon how easily a disease can spread from person to person. How are vaccines developed? Everyone now wants to have a little idea about this. You must be reading clinical trials phase 1, phase 2 with respect to the development of the COVID vaccine. Most vaccines have been in use for decades, with millions of people receiving them safely every year. As with all medicines, every vaccine must go through extensive and rigorous testing to ensure it is safe before it can be introduced in a country’s vaccine programme. Each vaccine underdevelopment must first undergo screenings and evaluations to determine which antigen should be used to invoke an immune response. This preclinical phase is done without testing on humans. An experimental vaccine is first tested in animals to evaluate its safety and potential to prevent disease. If the vaccine triggers an immune response, it is then tested in human clinical trials in three phases. Phase 1 The vaccine is given to a small number of volunteers to assess its safety, confirm it generates an immune response, and determine the right dosage. Generally, in this phase vaccines are tested in young, healthy adult volunteers. Phase 2 The vaccine is then given to several hundred volunteers to further assess its safety and ability to generate an immune response. Participants in this phase have the same characteristics (such as age, sex) as the people for whom the vaccine is intended. There are usually multiple trials in this phase to evaluate various age groups and different formulations of the vaccine. A group that did not get the vaccine is usually included in phase as a comparator group to determine whether the changes in the vaccinated group are attributed to the vaccine, or have happened by chance. Phase 3 The vaccine is next given to thousands of volunteers – and compared to a similar group of people who didn’t get the vaccine but received a comparator product – to determine if the vaccine is effective against the disease it is designed to protect against and to study its safety in a much larger group of people. Most of the time phase three trials are conducted across multiple countries and multiple sites within a country to assure the findings of the vaccine performance apply to many different populations. During phase two and phase three trials, the volunteers and the scientists conducting the study are shielded from knowing which volunteers had received the vaccine being tested or the comparator product. This is called “blinding” and is necessary to assure that neither the volunteers nor the scientists are influenced in their assessment of safety or effectiveness by knowing who got which product. After the trial is over and all the results are finalized, the volunteers and the trial scientists are informed who received the vaccine and who received the comparator. When the results of all these clinical trials are available, a series of steps are required, including reviews of efficacy and safety for regulatory and public health policy approvals. Officials in each country closely review the study data and decide whether to authorize the vaccine for use. A vaccine must be proven to be safe and effective across a broad population before it will be approved and introduced into a national immunization programme. The bar for vaccine safety and efficacy is extremely high, recognizing that vaccines are given to people who are otherwise healthy and specifically free from the illness. Further monitoring takes place in an ongoing way after the vaccine is introduced. There are systems to monitor the safety and effectiveness of all vaccines. This enables scientists to keep track of vaccine impact and safety even as they are used in a large number of people, over a long time frame. These data are used to adjust the policies for vaccine use to optimize their impact, and they also allow the vaccine to be safely tracked throughout its use. Once a vaccine is in use, it must be continuously monitored to make sure it continues to be safe. Vaccines are sensitive to biological products. Some are sensitive to freezing, some to heat, and others to light. Vaccine potency, meaning its ability to adequately protect the vaccinated patient, can diminish when the vaccine is exposed to inappropriate temperatures. It’s called the” cold chain”, is a system of storage and transport of vaccines at low temperature from the manufacturer to the actual vaccination site. This is an important component of any immunization program. Failures in the mass immunization program (occurrence of preventable diseases happens because of failure of cold chain system). One uses a chain of equipment for storage from manufacturers to the actual site and thus it's called a cold chain. Side effects can occur with any medicine, including vaccines. Depending on the vaccine, these can include a slight fever, rash, or soreness at the site of injection. Slight discomfort is normal and should not be a cause for alarm. COVID -19 Vaccine Status Scientists around the world are developing many potential vaccines for COVID-19. These vaccines are all designed to teach the body’s immune system to safely recognize and block the virus that causes COVID-19. Several different types of potential vaccines for COVID-19 are in development, including: Inactivated or weakened virus vaccines, which use a form of the virus that has been inactivated or weakened so it doesn’t cause disease, but still generates an immune response. Protein-based vaccines, which use harmless fragments of proteins or protein shells that mimic the COVID-19 virus to safely generate an immune response. Viral vector vaccines, which use a virus that has been genetically engineered so that it can’t cause disease, but produces coronavirus proteins to safely generate an immune response. RNA and DNA vaccines, a cutting-edge approach that uses genetically engineered RNA or DNA to generate a protein that itself safely prompts an immune response. As per the WHO Draft Landscape of COVID-19 candidate vaccines published on 12 November 2020 there are 48 candidate vaccines in clinical evaluation. Most of them are in various stages of clinical trials. They are offering 2 doses regimen and the timing is 0, 14 days or 0,21 days or 0,28 days. The route of administration in most of them is Intramuscular injection. Two to three candidates are to be launched by early 2021 as per reports in the media. There are 165 candidate vaccines in preclinical evaluation. It’s too early to know if COVID-19 vaccines will provide long-term protection. Additional research is needed to answer this question. However, it’s encouraging that available data suggest that most people who recover from COVID-19 develop an immune response that provides at least some protection against reinfection – although we’re still learning how strong this protection is, and how long it lasts. It’s also not yet clear how many doses of a COVID-19 vaccine will be needed and in what quantity. There are many strict protections in place to help ensure that COVID-19 vaccines will be safe. Like all vaccines, COVID-19 vaccines should go through a rigorous, multi-stage testing process, including large (phase III) trials that involve tens of thousands of people. These trials, which include people at high risk for COVID-19, are specifically designed to identify any common side effects or other safety concerns. If a clinical trial shows that a COVID-19 vaccine is safe and effective, a series of independent reviews of the efficacy and safety evidence is required, including regulatory review and approval in the country where the vaccine is manufactured, before WHO considers a vaccine product for prequalification. Part of this process also involves a review of all the safety evidence by the Global Advisory Committee on Vaccine Safety. An external panel of experts convened by WHO will analyze the results from clinical trials and along with evidence on the disease, age groups affected, risk factors for disease, and other information, will recommend whether and how the vaccines should be used. Officials in individual countries will decide whether to approve the vaccines for national use and develop policies for how to use the vaccines in their country based on the WHO recommendations. After a COVID-19 vaccine is introduced, WHO will support work with vaccine manufacturers, health officials in each country, and other partners to monitor for any safety concerns on an ongoing basis. Till the Vaccine is out, please follow COVID appropriate behavior i.e. Use Mask, Maintain Social Distance, Wash Hands with Soap at least 7 times a day, use hand sanitizer, avoid crowded places, go outside if only required and take care of the elderly and the sick. Stay safe. References: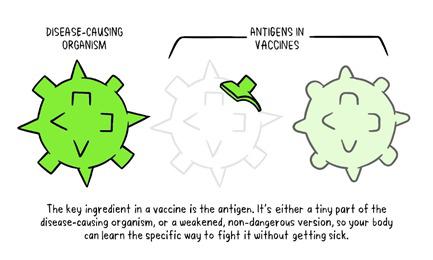
-Dr.Vishwanath Jagannath
-He completed MBBS from S.C.B Medical College, Cuttack and Fellowship in HIV medicine from the School of Tropical Medicine, Kolkata.
-Served Tata Steel for 15 years, last position held was Head, Centre for Family Initiatives, subsequently joined Jindal Steel and Power Ltd as Head, CSR.
-Thereafter joined Odisha Power Generation Corporation as DGM, CSR and R&R. Presently working with Odisha Coal and Power ltd.
-He can be contacted at vishyjagannath@gmail.com